The DESCAR-T registry has been created at the request of the French Health Authorities to collect data on the efficacy and safety at both short- and long-term of all CAR-T cell therapies marketed in France.
Cytokine Release Syndrome (CRS) is a major, potentially fatal, side effect of these therapies and improving their tolerance has been a major challenge.
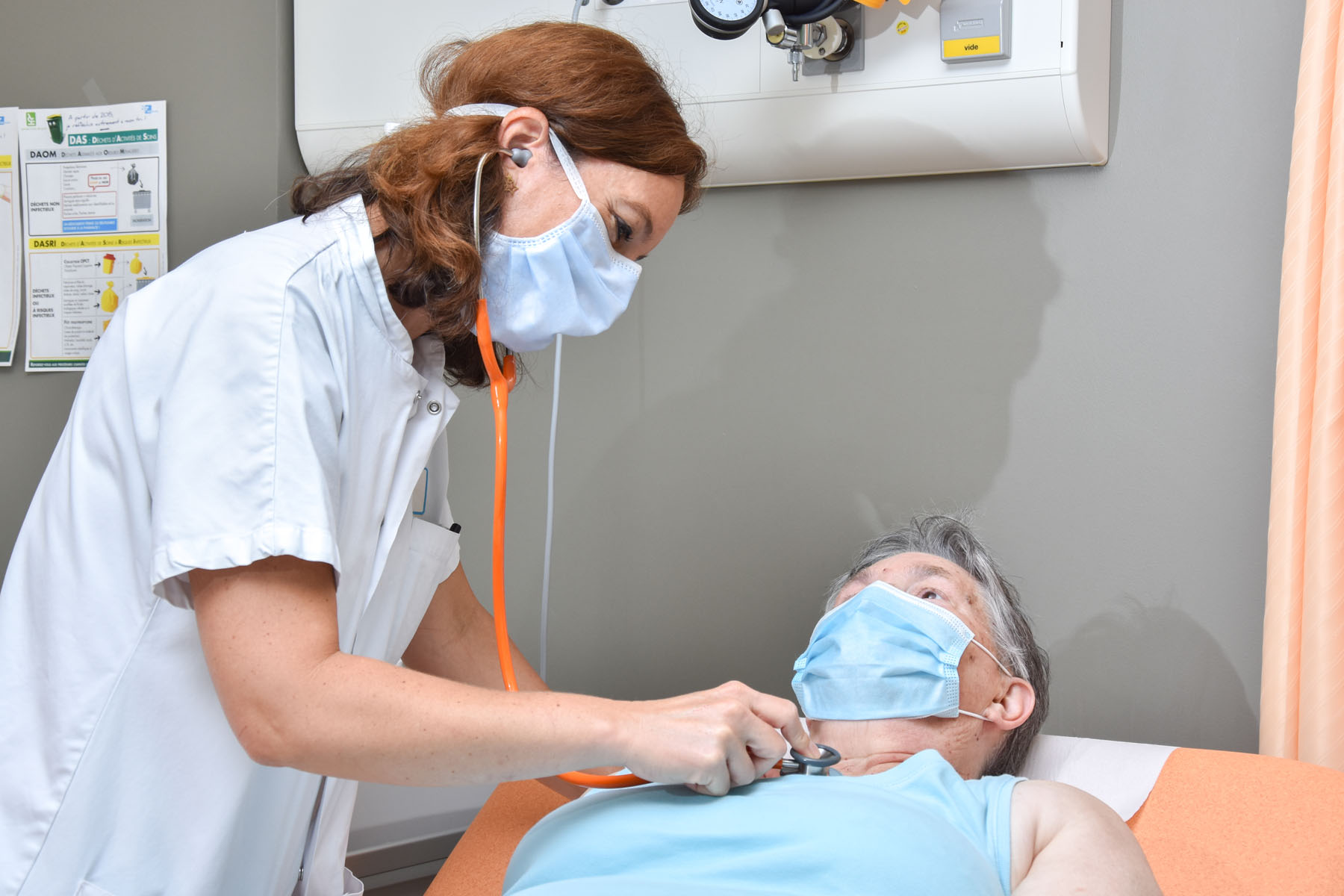
SYLVANT® (siltuximab) is commercialized by the pharmaceutical company EUSA Pharma, with an indication to treat multicentric Castleman’s disease in adults who are not infected with human immunodeficiency virus (HIV) and human herpesvirus-8 (HHV-8). Notably because of its mechanism of action, certain hematologists use it off-label to treat CAR-T-induced CRS, mainly in combination with other treatments.
In order to get insight on the use of Siltuximab in real-life settings, EUSA Pharma contacted the LYSARC to realize a project with the DESCAR-T Registry. The objective is to describe CAR T-cell-induced CRS, the profile of patients experiencing these adverse effects and their treatment in real life setting.
The analyses for this project will start in the course of the first trimester of 2023, with possible annual updates, and use the data of patients included in DESCAR-T until September of the previous year.
The LYSARC will perform the data analyses and the laboratory will only receive descriptive reports, where results are anonymized (i.e., it is not possible to identify any patient from the information in the report).
To the patients that received CAR-T, if you would like more information about the treatment of your data in this project or in the DESCAR-T Registry, you can contact the doctor at your centre of care for your CAR-T treatment or make a request at dpo@lysarc.org. You can find more details your rights regarding your data in the section “I am participating or have participated in a clinical trial“, or in the Patient Information Leaflet you received at the time of your inclusion in the registry.